Background: Lytic bone lesions are one of the most common clinical characteristics of patients with multiple myeloma (MM) and identification of bone lesions help distinguish between patients with smoldering multiple MM and active MM. Given this, the most recent update of the diagnostic criteria for MM incorporates advanced imaging approaches for distinguishing between the two entities. Several small retrospective studies have compared conventional skeletal survey (SS) with whole-body low dose computed tomography (WBLDCT) scan or the CT portion of a positron emission tomography (PET) scan. Conducting prospective studies comparing these two modalities side by side is limited by the radiation exposure. We undertook this study to provide a comparison between these two imaging modalities in terms of their ability to recognize lytic bone lesions in patients with MM.
Patients and methods: This was a retrospective study of consecutive patients with the diagnosis of MM treated at Mayo Cinic Hospital during 2004- 2018. Patients were included if they had a conventional skeletal survey no more than 3 months before MM diagnosis or any time after diagnosis and also had a WBLDCT or PET-CT within a 12-month period following the skeletal survey. We chose this approach since it is unlikely that a patient would have had both modalities done at the same time as part of standard of care (SOC). This approach was also facilitated by a gradual change in our SOC for skeletal imaging from conventional skeletal survey to WBLDCT. To measure the robustness of our findings, we performed a sensitivity analysis in patients who had the exam ≤6 months apart. Proportions were compared using a chi-square test and survival estimates were calculated using the Kaplan- Meier methodology and compared using log rank test.
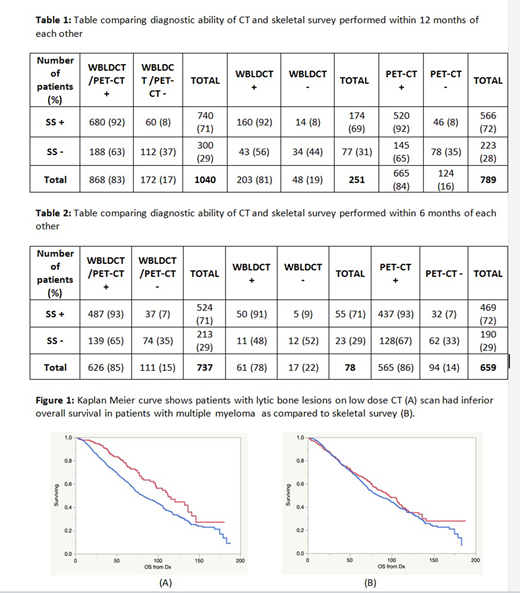
Results: The overall study cohort had 1040 patients, median age was 62 years at diagnosis (range, 24-94), 61% were male and 39% were female. The median time to the skeletal survey was 8 months from diagnosis (range, 0-160) and the median time to WBLDCT or PET-CT from SS was 2 months (0-12). A PET-CT was available in 789 (76%) of patients and WBLDCT in 251 (24%) of patients. Among the 300 patients with no lesions identified by SS, 188 (63%) had lytic lesions identified by CT, while in 60/740 (8%) patients with a positive SS did not have lytic lesions observed on the CT (p<0.0001). Overall, CT identified lytic lesions in 868 (84%) patients compared with 740 (71%) patients with lytic lesions seen on SS. Although the proportion of lytic lesions was slightly higher with PET compared to WBLDCT, the analysis did not reach statistical significance (65% vs. 56%; p = 0.17; Table 1a). After restricting the analysis to those with ≤6 months gap between low dose CT and SS (n=737), the result did not change (Table 1b). Further examination demonstrated that presence of lytic lesions by SS had no impact on OS from diagnosis, detection of lesions on the CT exam was associated with an inferior survival (112 vs. 81 months, p<0.0001). (Figure 1)
Conclusion: Skeletal survey has been the screening technique of choice for evaluation of bone involvement in MM for decades. However, CT based approaches have higher sensitivity with lytic lesions identified in a higher proportion of patients. The prognostic value of lytic disease is evident with CT based detection, again suggesting that this provides a more accurate estimate of the skeletal disease burden. Low dose CT rather than conventional radiographs should be the modality of choice for monitoring disease-progression and diagnosing active multiple myeloma.
Kapoor:Cellectar: Consultancy; Janssen: Research Funding; Sanofi: Consultancy, Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Celgene: Honoraria. Gertz:Abbvie: Other; Celgene: Other; Physicians Education Resource: Other: personal fee; Medscape: Other: personal fee, Speakers Bureau; Janssen: Other: personal fee; Spectrum: Other: personal fee, Research Funding; Johnson and Johnson: Speakers Bureau; Teva: Speakers Bureau; Annexon: Other: personal fee; Research to Practice: Other; Sanofi: Other; Proclara: Other; DAVA oncology: Speakers Bureau; Springer Publishing: Patents & Royalties; Appellis: Other: personal fee; Amgen: Other: personal fee; Prothena: Other: personal fee; Aurora Bio: Other; Alnylam: Other: personal fee; Ionis/Akcea: Other: personal fee. Dispenzieri:Takeda: Research Funding; Pfizer: Research Funding; Janssen: Research Funding; Intellia: Research Funding; Alnylam: Research Funding; Celgene: Research Funding. Dingli:Alexion: Consultancy; Janssen: Consultancy; Sanofi-Genzyme: Consultancy; Millenium: Consultancy; Bristol Myers Squibb: Research Funding; Rigel: Consultancy; Apellis: Consultancy; Karyopharm Therapeutics: Research Funding. Lin:Merck: Research Funding; Takeda: Research Funding; Legend BioTech: Consultancy; Juno: Consultancy; Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy; Vineti: Consultancy; Janssen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Sorrento: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gamida Cells: Consultancy. Kumar:Dr. Reddy's Laboratories: Honoraria; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Sanofi: Research Funding; Novartis: Research Funding; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Merck: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Carsgen: Other, Research Funding; Cellectar: Other; MedImmune: Research Funding; Karyopharm: Consultancy; BMS: Consultancy, Research Funding; Tenebio: Other, Research Funding; Genecentrix: Consultancy; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Kite Pharma: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal